A Cold-Chain Management Game Changer
Vaccines are fragile and require strict temperature controls to keep fresh and at full potency. The herculean journey from pharmaceutical warehouse freezers, to inoculation points across the United States, and finally to the arm of a patient, must be undertaken with great care for the cold chain to be effective. Implementing best practices and cold chain protocols are critical to ensure your vaccine arrives to the patient safe and at full strength. The problem is HOW? Cool Cube™ Vaccine Transport Coolers are easy-to-carry, keep vaccines safe and at the desired temperature, and they eliminate the need for ice or electricity. Cool Cube makes cold chain management easy, safe, and effective, and is a game changer for providers.
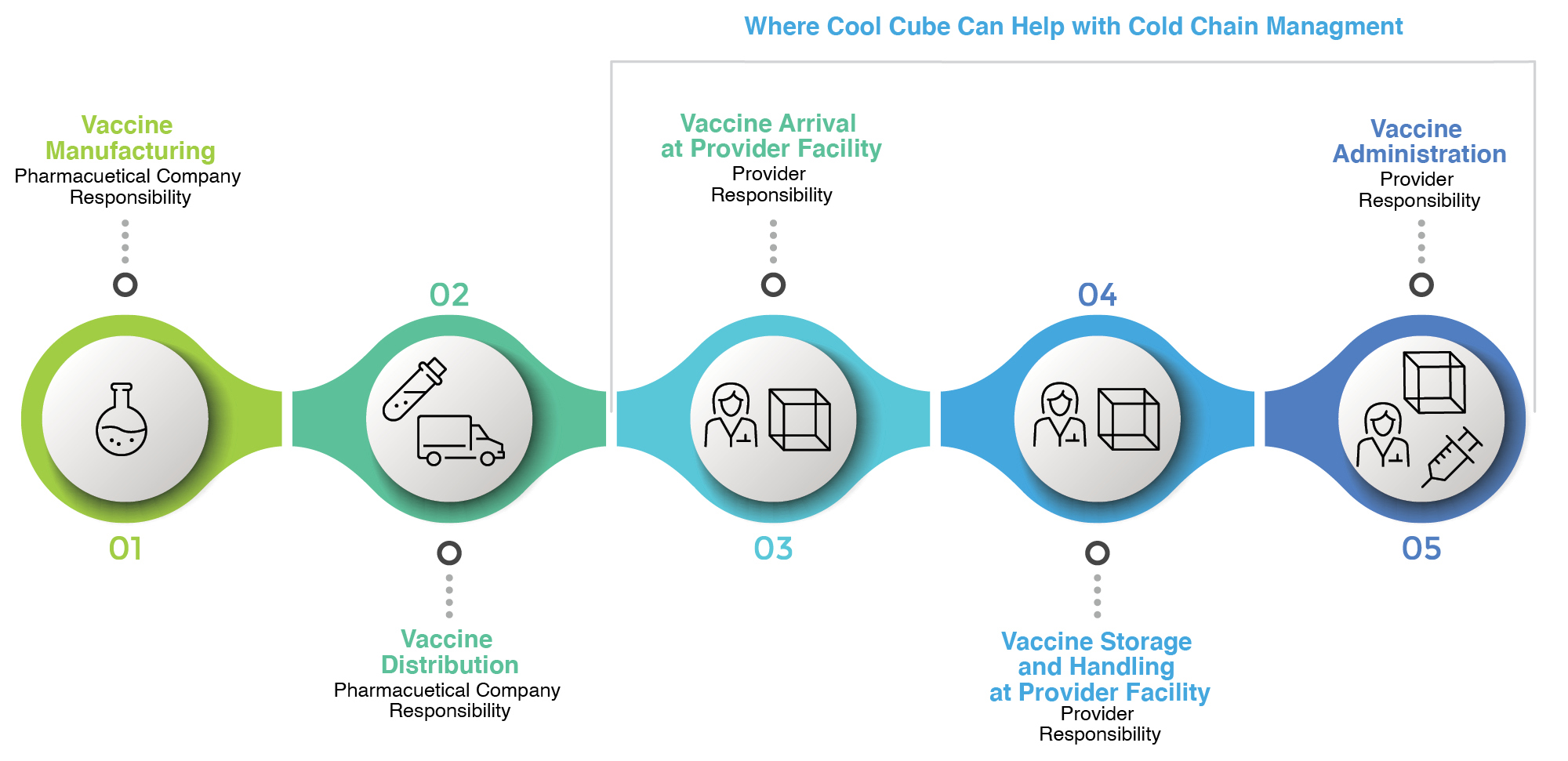
The Transportation of Vaccines
We all understand that the vaccine cold chain must be maintained during transport. The CDC recommends the use of portable refrigerators (with monitoring devices) for the transport of vaccines.
“If necessary, vaccines may be transported using a portable vaccine refrigerator with a temperature monitoring device placed with the vaccines. If a portable vaccine refrigerator is not available, qualified containers and packouts with a TMD in each container can be used.”
Source: U.S. Department of Health and Human Services Centers for Disease Control and Prevention, Vaccine Storage and Handling Toolkit, January 2020
| Emergency Transport | Transport for Off-Site Clinic, Satellite Facility, or Relocation of Stock | |
| Portable Vaccine Refrigerator or Freezer | Yes | Yes |
| Qualified Container and Packout | Yes | Yes |
| Conditioned Water Bottle Transport System1 | Yes | No |
| Manufacturer’s Original Shipping Container | Yes (last resort only) | No |
| Food/Beverage Coolers | No | No |
The Packing Vaccines for Transport during Emergencies1 tool describes a system in which properly conditioned frozen water bottles can be used as coolant when transporting vaccine during emergency situations. Source: https://www.cdc.gov/vaccines/pubs/pinkbook/vac-storage.html#transport
3 Ways Cool Cube Keeps Vaccines Cold
1.) Cool Cube™ Phase Change Material (PCM) is engineered to maintain a constant temperature at a specific melt/freeze point. When storing or transporting, PCM empowers Health Care Providers with a safe, effective means to maintain a desired temperature of vaccines.
PCM Features:
• Six panels for lab freezer temperatures included
• Patented, beveled-edge design seamlessly fit together
• Panels are interchangeable with other temperature systems
• Reusable (10,000+ cycles)
2.) Vacuum insulated panels offer advanced thermal protection far superior to other types of insulation. The temperature-holding qualities ensures the user a long-lasting, consistent temperature hold for days.
Vacuum Insulated Panel Features:
• Reinforced, clear-plastic, protective wrap
• Rigid structure is durable and light
• 5x the resistance (R-value) of conventional insulation
• Removable and replaceable.
3.) Each outer case is built with mobility in mind. Various features enable the user to move with confidence and know the contents are well protected (and at the correct temperature) during transit and use.
Easy, Safe Pack-Out
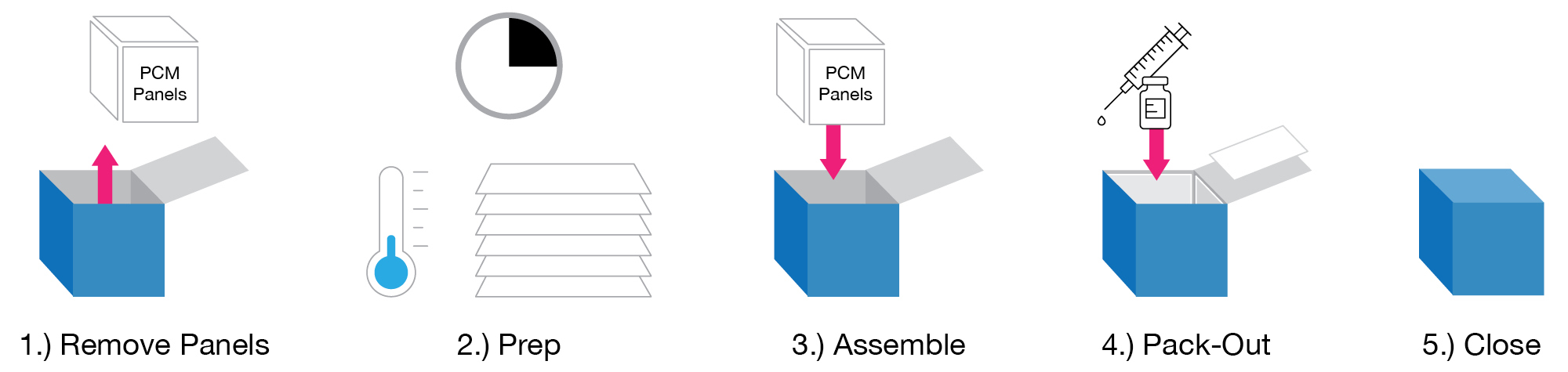
Cool Cube Sizes
 |
 |
 |
 |
| Cool Cube 03 | Cool Cube 08 | Cool Cube 28 | Cool Cube 96 |
|
65+ Hours Capacity Inside Dimensions Weight |
76+ Hours Capacity Inside Dimensions Weight |
103+ Hours Capacity Inside Dimensions Weight |
126+ Hours Capacity Inside Dimensions Weight |
Stabilizing the Temperature of Stored and Transported Vaccine
The use of a Digital Data Logger (DDL) is recommended by the CDC to insure the temperature of vaccines.
“CDC recommends a specific type of TMD called a“digital data logger” (DDL). A DDL provides the most accurate storage unit temperature information, including details on how long a unit has been operating outside the recommended temperature range (referred to as a“temperature excursion”). Unlike a simple minimum/maximum thermometer, which only shows the coldest and warmest temperatures reached in a unit, a DDL provides detailed information on all temperatures recorded at preset intervals.”
Source: U.S. Department of Health and Human Services Centers for Disease Control and Prevention, Vaccine Storage and Handling Toolkit, January 2020
Evolve Recommends The Vericor Traceable Memory-Lo Temperature Monitoring Data Logger Kit
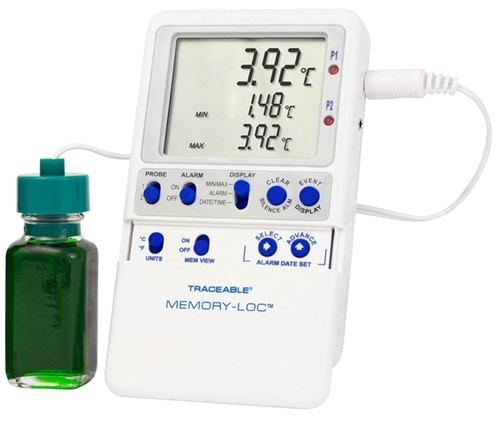
Vericor Traceable Memory-Lo Temperature Monitoring Data Logger Kitlocks over 1 million temperature observations (good for 21 CFR 11 environments). It is a calibrated logger that enables user-defined time intervals (1-minute to 24-hours), downloads data to any USB stick, and meets current CDC requirements making it ideal for monitoring temperatures while storing and or transporting vaccines. The data logging feature logs 1,051,200 data points. Base unit, probe, certificate, flip-open stand, wall mounting, Velcro®, magnetic strips, batteries, and universal adapter included.
Features
- Meets current CDC requirements for vaccine storage and monitoring (CDC Toolkit – January 2018)
- Data is transferred from thermometer to computer using any USB flash drive. No software needed
- Probe bottle (filled with nontoxic GRAS glycol) insulates sensor from momentary temperature changes
- Ten-foot micro-cable permits refrigerator doors to close on it
- Great for 21 CFR 11 environments to lock raw data and prevent clearing/changing on base unit
- Smart-Alarm™ features a visual/audible alarm that continues to alarm even if the unit returns to non-alarm conditions
- Hassle-free retrieval of data – unit can remain in use while downloading and analyzing data
- Status indicators – Low battery, memory full, USB data transfer and active alarm state
- User-defined data logging interval of one observation/minute to one observation/24 hours
- High-accuracy of ±0.25°C with a resolution of 0.01
- Multi-point calibration on an individually-numbered certificate assures accuracy
________________________________________________________
Resources:
Read the CDC article titled, Vaccine Storage and Handling
Download the CDC Vaccine Storage and Handling Toolkit
