Proudly manufactured in the USA
Positive Pressure
Patient Protection CAPSULSTM can be operated in positive pressure mode to protect an enclosed patient from outside contaminants. Isolator overpressure is developed by a powered air supply at its head end.
Negative Pressure
Patient Containment CAPSULSTM can be operated in negative pressure mode to enclose and isolate a contaminated patient, preventing cross-contamination to the outside. A powered air exhaust at its foot end develops isolator under-pressure.
Patient Airflow
Airflow to the patient in positive pressure mode (or from the patient in negative mode) is provided by a battery-powered PAPR (powered air-purifying respirator) blower fitted with CBRN primary filters. The 4 cfm rated blower provides 17 to 21 air changes per hour within the isolator depending on patient volume. Regardless of operating mode, patient airflow is always supplied at the head end exhausted at the foot end. Blower run time on a replaceable, single-use BA5800/U lithium battery is approximately 7 to 10 hours.
Primary Filtering
The primary filters for removal of contamination are CBRN (chemical, biological, nuclear, radiological) filter canisters similar to the military C2A1 and are mounted on the blower input ports. These canisters provide charcoal filtering of chemicals and chemical warfare agents (CWAs) and particulate filtering to HE/P-100 standards (99.97% @0.3µ).
Backflow Control
Check valves and/or HE/P-100 secondary filters prevent backflow of air and potential cross-contamination at the air inlet or exhaust ports that are not connected to the blower.
Particulate Barrier
ISOVAC’s Iso-ShellTM engineered plastic film shell material, pressure-seal zipper, and Iso-WeldTM seaming technology, together form a gas and liquid-tight envelope, providing a solid barrier to biological and radiological particulates. Barrier redundancy is achieved through the use of over or under pressurization of the envelope. The CAPSULSTM barrier system has undergone testing by U.S. military agencies responsible for chemical and biological defense.
Seaming Technology
ISOVAC’s proprietary Iso-WeldTM seaming technology fuses materials together to produce a redundant and 100% gas and liquid tight seam that is stronger than the materials alone.
Closure Technology
The CAPSULSTM zipper is based on technology originally developed for NASA space suits. The zipper is attached to the CAPSULSTM’ Iso-ShellTM envelope barrier using the same proprietary Iso-WeldTM seaming technology. CAPSULSTM zippers provide a gas and liquid-tight seal and undergo 100% pressure testing by their manufacturer.
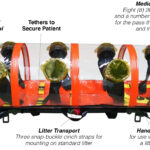
Litter Transport
CAPSULSTM has three snap-buckle cinch straps for mounting it on a standard litter. The litter provides a stable platform to lift and carry a patient without undue stress on the barrier envelope, and is required for negative pressure operation. This also allows CAPSULSTM to be transported within any medical evacuation vehicle (ambulance, aircraft, or ship) already capable of securing a litter, without vehicle modification or additional equipment.
Easy Setup
A CAPSULSTM PIU can be unpacked from its shipping box or carry bag, unfolded, attached to a litter, setup, and loaded with a patient by two trained personnel in about fifteen (15) minutes. The required litter can be supplied by the end-user or purchased from ISOVAC at additional cost.
Easy Load
The CAPSULSTM “suitcase” style opening allows unrestricted access to its interior for ease of patient loading and connection of end-user supplied medical equipment.
Easy View
The CAPSULSTM clear barrier envelope enables visual monitoring of the entire patient and provides the patient with an unrestricted view of his/her surroundings.
Patient Tethers
CAPSULSTM incorporates a versatile system for securing a patient including multiple individual leg straps, a waist strap, a multi-point upper torso harness, and wrist straps. The number and locations of the straps can be adjusted to accommodate a wide range of patient sizes and types of injury.
Medical Intervention
CAPSULSTM is fitted with 8 glove arms (4 per side) to facilitate patient treatment, and is equipped with a number of medical access ports of various sizes for the pass-through of medical tubing and instrument leads.
Durability
The CAPSULSTM proprietary Iso-ShellTM barrier materials offer a minimum improvement of ten-fold in their abrasion, puncture, and tear resistance over PVC-based materials of the same thickness. A reinforced base mat attached to the barrier envelope serves as a skid plate for added durability and protection, and provides attachment points for the carry straps and litter tethers.
Extended Temperature Range
The CAPSULSTM proprietary Iso-ShellTM barrier materials and zipper offer an operating temperature range greatly exceeding that of PVC materials. They remain flexible and user-friendly from -40˚F to 140˚F. The blower battery has a specified operating range of from -26˚F to +120˚F.
Decontamination
The CAPSULSTM proprietary Iso-ShellTM barrier materials, Iso-WeldTM seaming technology, gas and liquid-tight sealing zipper, coated carry straps, and glove-arm caps provide a smooth, non-absorbent surface that is free of pockets and crevices that might allow entrapment of CWAs, contaminants, and decontamination agents. This greatly improves the efficiency and efficacy of the decontamination process.
Fluid/Waste Control
Primary control of patient waste is intended to be via end user supplied adult diapers and/or urinary catheters, and press and seal waste bags. Secondary control of body fluids can be accomplished with end-user supplied absorbent pads placed under the patient.
Ancillary Parts
Ancillary parts and materials include with CAPSULSTM are a user’s manual, a blower user’s manual, a waterproof patient ID card with wire tie and marker, a blower outlet hose and air flow indicator, and a set of HE/P-100 filters.
Carry Bag
CAPSULSTM and all of its components are supplied packed in a carry bag for ease of transport.